As a leading global upstream enterprise in the in vitro diagnostics platform, DIA-UP has been deeply engaged in core biological raw materials for reagents for more than a decade and is a professional provider of special protein raw materials. The self-developed high-quality raw materials have undergone repeated testing and multi-platform verification, and a more systematic and rigorous process and management system are adopted for effective quality control. The first-generation intrinsic factor protein independently developed by DIA-UP has been widely praised by domestic and foreign customers since its launch. Through the continuous exploration and iterative optimization of the core technical team, a brand-new generation of intrinsic factor protein has been newly launched, which can be flexibly applied to multiple platforms such as fluorescent chromatography and chemiluminescence. After multiple steps of column chromatography, 1mol of intrinsic factor can bind to >0.95mol of VB12, with higher sensitivity, higher binding capacity, and better reagent performance. At the same time, Deaoping has established a safer and more stable supply chain, with stable delivery time and rapid response, which can minimize the time cost of customers and efficiently promote the stable development of experimental work.
Physical Properties:Clear and transparent
Source::Mammalian cells
Buffer System::Water
Purity:Greater than 97% by HPLC
Filtration:0.22um
Application Scope:suitable for the development of VB12-related reagents
Storage Conditions:For long-term storage, freeze at -20°C or below, and avoid repeated freezing and thawing
NO.2 IF Bridging Antibody
In the development of high-difficulty luminescent reagents for VB12, the commonly used pairing is a combination of intrinsic factor protein (IF) and VB12 hapten. However, experimental procedures have shown that when intrinsic factor protein (IF) is coated onto magnetic beads, the binding capacity of the intrinsically active IF to VB12 in samples decreases. The cause of this phenomenon is that the covalent coupling of IF to magnetic beads induces structural changes in IF, which significantly affects its affinity for VB12 in samples.
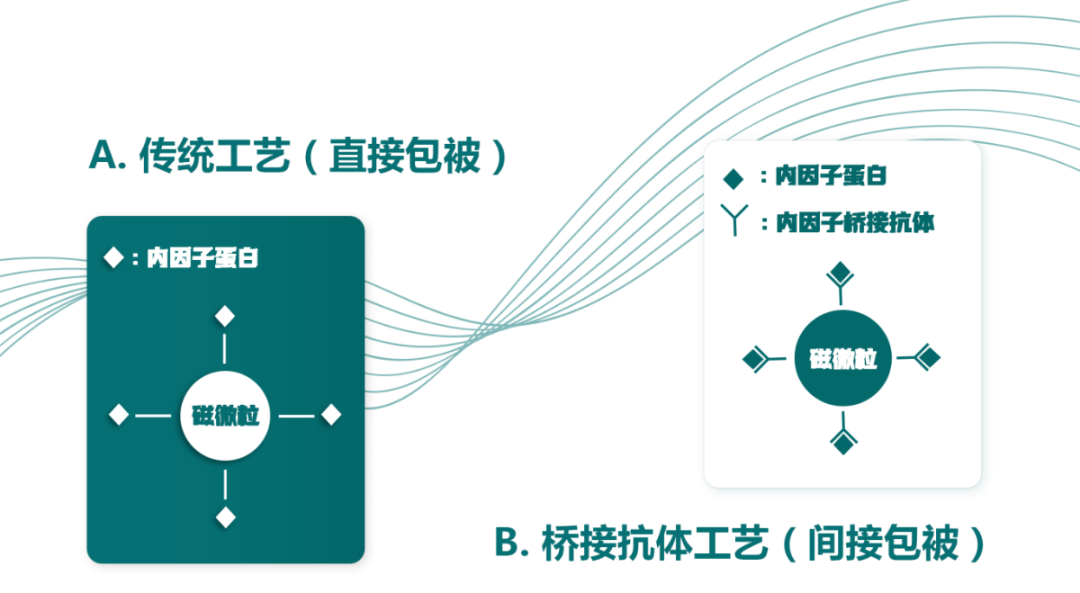
In response to this issue, the core technical team of DIA-UP has independently developed an intrinsic factor magnetic bead coating tool - the intrinsic factor bridging antibody. It achieves indirect coupling between the intrinsic factor protein (IF) and magnetic beads. On the one hand, it can efficiently avoid structural changes caused by covalent coupling between IF and magnetic beads, completely preserving the high binding activity of IF to VB12. On the other hand, it can significantly reduce the dosage of intrinsic factor, helping customers effectively reduce the development cost of VB12 reagents and facilitating the production optimization of high-difficulty reagents.
· Performance Verification Report - Chemiluminescence Platform
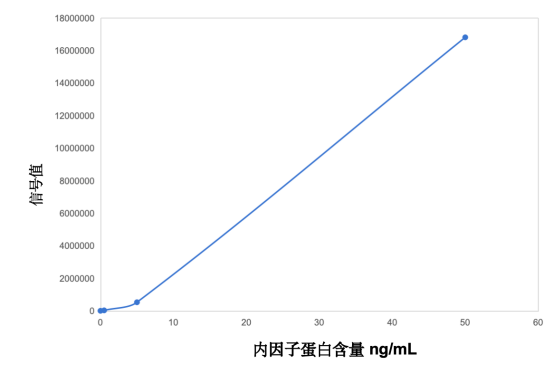
When the IF bridging antibody is coated on magnetic beads and different concentrations of intrinsic factor protein (IF): 0-0.5-5-50 ng/mL are added, it shows a good dose-response, proving that it can effectively bridge the IF protein.
· To further confirm whether the binding site of the IF bridging antibody to the intrinsic factor protein would interfere with the binding of VB12, the following experimental protocol was designed:
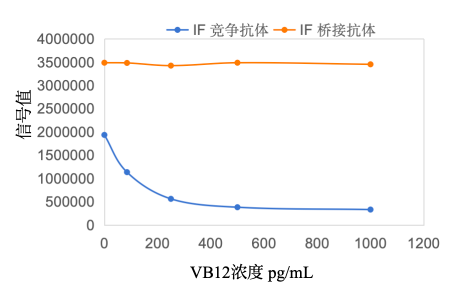
01. The IF bridging antibody showed no change in reaction signal values with the increase of VB12 concentration, indicating that the binding sites of VB12 and the IF bridging antibody on the intrinsic factor protein do not interfere with each other.
02. The IF competitive antibody showed a continuous decrease in reaction signal values with the increase of VB12 concentration, indicating that there is a competitive reaction between VB12 and the IF competitive antibody at the binding site on the intrinsic factor protein.
NO.3 IF Bridging Antibody
Non-site-specific coupling products easily cause problems such as masking and destruction of many epitopes, as well as homogeneity of coupling products, affecting the final sensitivity and stability of reagents. Based on this, DIA-UP strengthens its continuous innovation capability and continuously expands the SDC site-specific conjugate product line to help customers easily solve the series of problems caused by non-site-specific coupling. As Deaoping's top-tier advantageous raw material project, intrinsic factor (IF) has now launched a full set of raw material solutions for the development of high-difficulty reagents VB12 and active-VB12. Among them, the SDC site-specific conjugate - intrinsic factor (IF)-ALP protein-enzyme conjugate can achieve better signal-to-noise ratio and extremely high sensitivity at low concentration. It helps customers easily achieve directional coupling of proteins and alkaline phosphatase, and efficiently assists in the production optimization of local chemiluminescent products.
Better Signal-to-Noise Ratio • Extremely High Sensitivity • Extraordinary Stability
Generally, the intrinsic factor (IF)-ALP conjugates prepared based on SMCC/SATA and other schemes have heterogeneity,
and may damage the VB12 binding site on the intrinsic factor. The connection site of intrinsic factor IF-ALP (SDC) is far away
from the VB12 binding domain.
When DIA-UP's intrinsic factor IF-ALP is used to replace the corresponding component of commercial VB12-AP reagents,
it shows a better signal-to-noise ratio and sensitivity, and the usage concentration of the conjugate is only 5 ng/mL.
Thermal Accelerated StabilityMeanwhile, the thermal accelerated stability of DIA-UP's intrinsic factor IF-ALP was further evaluated.
When stored at 37°C for 7 days at a concentration of 5 ng/mL, the activity retention rate exceeded 90%.
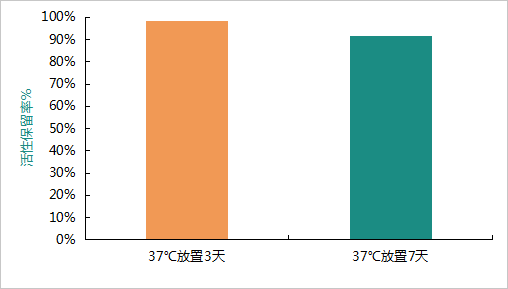
BEIJING DIA-UP BIOTECHNOLOGIES CO.,LTD
Sales hotline:+86185 1363 7053
Service hotline:+86185 1363 7192
After-sales hotline:+86185 1363 7531
Order Email:order@dia-up.com
After-sales Email:service@dia-up.com
Official website:www.dia-up.com
Company Add:Building 18, No. 2, Huankexizhong Road, Tongzhou District, Beijing